- Prologue
- Water: Importance in Biosphere
- Hydrogen Peroxide (H2O2)
- Water gas / Syngas
- Acid, Base, Salts
- Extensive vs. Intensive properties
- Misc Terms
- Possible MCQs
Prologue
- Continuing on…This article contains revision note out of Chapter 6, 7, 8 and 9 of Chemistry Class11.
- As such nothing much from 6 to 8 because they deal with technical stuff in thermodynamics, redox equation etc.
Water: Importance in Biosphere
- Water plays a key role in the biosphere because compared to other liquids, water has a higher specific heat, thermal conductivity, surface tension, dipole moment and dielectric constant.
- Water moderates climate and body temperature because of its high heat of vaporisation and heat capacity are responsible.
- Water is an excellent solvent for transportation of ions and molecules for plant-animal metabolism.
- ice cube floats on water because Ice’s density is less than that of water..
- In winter season ice formed on the surface of a lake gives thermal insulation & helps aquatic life to survive.

Possible MCQ: Ascending descending order of water sources)
Water: Chemical Properties
- Water can dissolve many salts, particularly in large quantity, makes it hard and hazardous for industrial use.
- The polar nature of water makes it: (a) a very good solvent for ionic and partially ionic compounds; (b) to act as an amphoteric (acid as well as base) substance; and (c) to form hydrates of different types.
- Amphoteric Nature: Water can act as an acid as well as a base.
- Extensive hydrogen bonding between water molecules= high freezing point, high boiling point. And Due to this hydrogen bonding with polar molecules, even covalent compounds like alcohol and carbohydrates dissolve in water.
- Rain water is almost pure (except some dissolved gases from the atmosphere)
- When rain water flows through earth surface, it dissolves many salts and becomes hard.
- Soft water: it doesn’t have calcium and magnesium’s soluble salts. Soft water gives lather with soap easily.
Hard Water: Properties
- Hard water does not give lather with soap.
- Presence of calcium and magnesium salts in the form of hydrogencarbonate, chloride and sulphate in water makes water ‘hard’.
- Soap containing sodium stearate reacts with hard water to precipitate out Ca/Mg stearate.
- Hard water forms scum/precipitate with soap, so it is unsuitable for laundry.
- This salt deposit also decreases efficiency of boiler.
Hard water: Types & Treatment
- Hardness of water is of two types: (i) temporary hardness, and (ii) permanent hardness.
| Temporary hardness | Permanent Hardness |
|---|---|
| Due to the presence of magnesium and calcium hydrogen- carbonates. | Due to presence of presence of soluble salts of magnesium and calcium in the form of chlorides and sulphates in water. |
Removed by following methods:
|
Not possible to remove this hardness by boiling. But following methods will work
|
Hydrogen Peroxide (H2O2)
| Isotop | proton | neutron | rarity |
| protium | 1 | 0 | 99.985% hydrogen is like this |
| Deuterium (heavy hydrogen) | 1 | 1 | 0.015% |
| Tritium (radioactive) | 1 | 2 | trace amount in earth |
Hydrogen paroxide: chemical properties
- Pollution control treatment of domestic and industrial effluents.
- Properties: Colourless-pale blue liquid. Acts as an oxidising as well as reducing agent in both acidic and alkaline media.
- H2O2 decomposes slowly on exposure to light hence stored in dark.
- In metal surfaces or traces of alkali (present in glass containers), H2O2 reaction is catalysed. Therefore it is stored in wax-lined glass or plastic vessels in dark.
- Urea can be added as a stabiliser.
- H2O2 must be kept away from dust because dust can induce explosive decomposition of the compound.
H2O2: Uses
- Hair bleach, bleaching agent for textiles, paper pulp, leather, oils, fats, etc.
- Mild disinfectant, antiseptic (perhydrol).
- Sodium based detergents.
- Synthesis of pharmaceuticals (cephalosporin, hydroquinone), tartaric acid and certain food products
- Used in Environmental (Green) Chemistry- for pollution control treatment of domestic and industrial effluents, oxidation of cyanides, restoration of aerobic conditions to sewage wastes, etc.
Water gas / Syngas
- Water gas/syngas/synthesis gas is the mixture of CO and H2.
- It is further used for the synthesis of hydrocarbons such as methanol.
- Syngas is produced from sewage, saw-dust, scrap wood, newspapers etc.
- Coal gasification is the process of producing ‘syngas’ from coal.
Dihydrogen Properties and uses
- Dihydrogen is a colourless, odourless, tasteless, combustible gas.
- Prepared by (1) water-gas shift reaction from petrochemicals (2) electrolysis of brine.
- Lighter than air and insoluble in water.
- Dihydrogen =>Synthesis of ammonia =>mfg. of nitric acid and nitrogenous fertilizers.
- hydrogenation of polyunsaturated vegetable oils like soyabean, cotton seeds etc=> Vanaspati Ghee.
- Manufacture of methanol and other bulk organic chemicals.
- Mfg. of metal hydrides, hydrogen chloride.
- To reduce heavy metal oxides to metal
- Atomic hydrogen and oxy-hydrogen torches used for cutting and welding purposes. They can generate the temperature of 4000 K.
- Dihydrogen fuel cells = electrical energy and rocket fuel.
Hydrogen Economy: basis principle
- use di-hydrogen to store and transport energy.
- Energy is transmitted in the form of dihydrogen and not as electric power.
- 2005: India launched pilot project to use dihydrogen as fuel for running automobiles.
- Initially 5% dihydrogen has been mixed in CNG for use in four-wheeler vehicles. It’ll be increased gradually.
- dihydrogen can release 3x times more energy than petrol
- It produces less pollutants than than petrol.
- Dihydrogen’s pollutant is called “oxides of dinitrogen”. We can minimize its emission by adding water in the cylinder.
Limitations of Dihydrogen fuel
- Its cylinder weight 30 times more than petrol tank.
- Liquification requires cooling to 20K. This Is very expensive.
Acid, Base, Salts
| Acid | Base |
|---|---|
| turn blue litmus paper into red | turn red litmus paper blue |
| Acid accepts electron pair | Donates pair of electrons |
| “acid” word derived from a latin word “acidus” meaning sour. | Bases are known to , taste bitter and feel soapy. Common example: washing soda. |
|

Possible MCQ: Ascending-Descending order
| Hydrochloric acid | Gastric juice essential for digestions |
| Acetic acid | main constituent of vinegar. |
| Lemon juice | citric acid |
| Orange Juice | ascorbic acids |
| Tamarind paste | tartaric acid |
Salts
- When acids and bases are mixed in the right proportion they react and give salts.
- Example: sodium chloride, barium sulphate, sodium nitrate.
- hydrochloric acid + sodium hydroxide.= Salt (Sodium chloride)
PH
- pH scale: Hydronium ion concentration in molarity – that is expressed on a logarithmic scale known as the pH scale.
- Buffer Solutions: These solutions resist change in pH in presence of acid or alkali.
- The pH of a solution is defined as the negative logarithm to base 10 of the activity
- PH Measurement has biological and cosmetic applications.
- Now-a-days pH paper is available with four strips on it. They can determine PH with an accuracy of ~0.5.
- pH meter is a device that measures the pH-dependent electrical potential of the test solution within 0.001 precision.
- pH meters of the size of a writing pen are now available in the market

Extensive vs. Intensive properties
| extensive properties | intensive properties |
|---|---|
| Depends on quantity of the matter in system | Those properties which do not depend on the quantity or size of matter present |
| Examples: mass, volume, internal energy, enthalpy, heat capacity, etc. | Temperature, pressure, density |
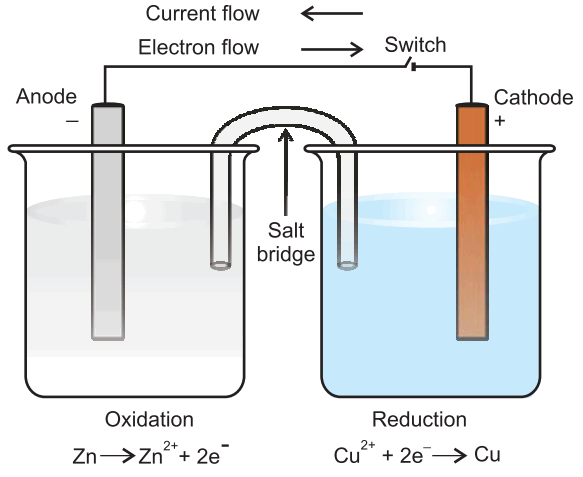
Daniell cell: Electrons produced at the anode due to oxidation of Zn travel through the external circuit to the cathode where these reduce the copper ions.
Possible MCQs
- match the following: (1) methods to remove hardness of water vs. chemical principle (2) isotopes of hydrogen (3) chemical vs. its uses (4) food item vs. acid component
- assertion reasoning related to (1) water’s importance in biosphere (2) why water used in dihydrogen engine
- which of the following statements are correct about (1) ice (2) hard water and its treatment methods (3) hydrogen
- Arrange following xyz items in their ascending / descending order of PH.

![[Science WMD] ISRO POEM, NASA CAPSTONE, GEMCOVAC-19, Doctors Day, Weekly Mrunal Digest from Jun week4-2022](https://mrunal.org/wp-content/uploads/2022/07/isro-poem-scaled-500x383.jpg)
![[Revision] Agriculture Chemistry: Mineral Nutrition, Plant Growth Regulators, Ethylene, Hydroponics, Photoperiodism, Vernalisation](https://mrunal.org/wp-content/uploads/2015/08/c-nitro-cycle-500x383.jpg)
![[Revision] Chemistry Part-2: Metal, Non-Metal, Metalloid, Liquid, Surface tension, Viscosity, Gas Laws, Periodic Table](https://mrunal.org/wp-content/uploads/2015/08/c-elementbl-500x383.gif)
Very nice stuff. Thanks !
Please upload current affair update of excel file. it won’t take more than two days for you sir. In prelims they are directly and indirectly asking question from those topics only. Please Sir !
tan q very much mrunal
Sir, aap hindi medium me bhi article likhne ki bhi kirpaa karen.
Thanks a ton man !
thank u very much sir for all revision notes………….
Thank u.. This General Science especially Chem and Bio is very crucial for CSE Preliminary Exam
Pls complete remaining Lectures….. At least reply whether you are doing them or not ? I request to do Lectures for Mains as well. No amount of article writings will help the aspirants and make the site popular… when compared to lectures.
Continue what ur doing…….u don’t know much it is helping us who lives in village and cant afford coaching
sir thanks 4 this article . i request u 2 upload history & biology videos if possible it will be of immense help
sir thanks alot for this essential articles of Chemistry. sir do it for Bio & art and culture also,if possible.
Great job team mrunal. Keep uploading similar material for other subjects too fron Prelims perspective.
How can H2O2 be colorless pale blue? Either it can be colorless or Pale blue. Please check
H202 is colourless gas and pale blue in liquid state.
Thanks for wonderful article!!!
A good one thank-you sir
Mrunal sir,please update current affair.if you can’t atleast intimate us.lots of us depend on u for current affair. Pt exam is imminent need your reply urgently.
Thank you so much:)
Could anyone give overview of PDS Santa kumar recommendation ?
sir plz provide help notes in history too
Thank you sir
superb …. Great work … much appreciated
Please recheck pH of soft drinks—> should be ~6.0
sameer. it cant be 6.0 . check it once more. it has to be around 3.
plz any experienced candidate guide up to which date one shud prepare current affairs?
Sir plz give all current affair science tech important topics for prelims 2015
sir where are the part 1 and part of chemistry?
THanks for this write up. its of immense help.
Hi. Mrunal sir!
I gave CGL-tier 1 exam on 16-aug-2015. The code provided on the OMR sheet did not match with the code which was provided on the question paper. I asked the invigilator. Even they were in dilemma. some how they managed and made us bubble the code given on question paper.
But my doubt is, code provided on OMR sheet and code on question paper did not match.
Mrunal sir kindly address my query.
Sir please give some strategies for new pattern of RBI grade b
Sir,
Baat ye jai ki English bounce ho jati jai aur apke article English me hi aate hai.isliye agrah hai ki ek website Hindi me bhi launch kariye .apke article shandar hote hai .English me padhne se adha samjh me at a h adha upr se nikl jata h…so plzz help me..
Thank you!
water treatment related books
Boiler Water Treatment