- What are DNA and Gene?
- What are Transgenics?
- Why do we need GM technology?
- Current issue:
- Recommendations of the committee:
- What is this GEAC:
- Procedure to accord approval:
- Convention on Biological Diversity (CBD)
- Cartagena Protocol on Biosafety (CPB)
- Nagoya Protocol on Access and Benefit Sharing (ABS)
What are DNA and Gene?
- Always a human gives birth to humans, and a baby is said to inherit his/her parents’ characters. This is made possible only because of the so called genes. Genes are the books where all your personal, family characters are coded passed on to you from your parents.
- DNA is a polymer of nucleic acid specifically deoxyribonucleic acid which is in turn is comprised of sugar component and nitrogenous base.there are 4 type of nitrogenous base that is adenine , guanine, cytosine and thiamine. It is the sequence of theses nitogenous bases that determine our genetic charecter. [courtsey- Ritesh’s comment]
- DNA aids in protein synthesis.
- Their expression gives you characters.
What are Transgenics?
- Scientists have now mapped, analyzed these genes of various plants and animals. i.e., understood the ABC of gene make up
- Here now they are capable of manually rearranging these genes, inserting a part, deleting one changing the way one behaves, like stunted coconut trees giving coconuts at your arms stretch.
- Such organisms are called TRANSGENICS, transformed genetic make-up.
Why do we need GM technology?
- Already a population of 7 billion and growing energetically.
- 3% increase in agri production needed to ensure food security to this population, while current growth rate is only 2%.
- Stress on land resource already huge- use of insecticides, pesticides.
- 27% of world’s undernourished people proudly are Indians- Urgent need to feed those hungry mouths. Else National shame will be the result- as our PM remarked on the HUNGAMA report.
To address above problems GM aids the development of specific traits in crops like:
- Herbicide resistance
- Pest resistance
- Viral resistance
- slow-ripening
- Fungal and bacterial resistance
- Quality improvement (protein and oil)
- Value addition (Vitamins, micro-and macro-elements)
Ok then let us move quick into this akshayapaatra Hold on, every coin has one more side:
- Biosafety first concern
- Direct health effects (toxicity)
- The stability of the inserted gene
- Nutritional effect associated with genetic modification
- Any unintended effects which could result from the gene Insertion.
- Autonomy of farmers affected because the seeds of these tech crops are monopolized and are marketed by big private firms. If he is unable/does not provide us the requisite seeds, we have a problem.
- Genetic erosion of our local varieties.
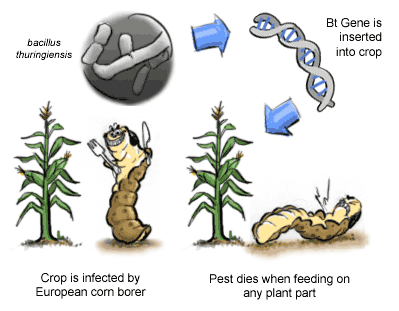
Current issue:
- In India it all started with Bt cotton and Bt Brinjal, regarding control, regulation, marketing production, safety of such crops.
- Here the Bt refers to Bacillus thuringenesis soil bacterium from which the genes are introduced in to the native cotton and brinjal varieties.
- The gene gave an expression in cotton which produced a protein in the cotton crop that was toxic to the boll worms and stem borers (pests) i.e., pest resistant variety.
- Now the issue with us already with many political parties, farmers complaining introduction such GM cotton, Brinjal(stopped after initial intro) as the cause for increasing farmer suicides in Karnataka, Vidharbha region. High input cost of seeds, genetic erosion of local varieties, farmer’s dependence on private seed cos are said to be the reasons.
In this scenario our parliamentary committee on Agriculture has submitted a report on CULTIVATION OF GENETICALLY MODIFIED FOOD CROPS – PROSPECTS AND EFFECTS
Recommendations of the committee:
- No GM food trials, till a stronger regulatory system is established.
- Current regulation by GEAC (Genetic Engineering appraisal committee) flawed, GEAC under dept. of Biotech which has one of its aims to spread modern biotech. One cannot regulate his own product is the reports’ view.
- Farmers of cotton already under strain, new Bt cotton seeds are expensive, input costs high, yet farmers are left with zero choice.
- Strict labeling giving choice for consumers to know what they buy is must. Especially imported foods are now a concern.
What is this GEAC?:
- Established under MoEF,
- GEAC is the apex body to accord approval of activities involving large scale use of hazardous microorganisms and recombinants in research and industrial production from the environmental angle.
- GEAC is also responsible for granting approvals relating to release of genetically engineered organisms and products into the environment including experimental field trials.
- The GEAC also has the powers to prohibit, revoke, supervise and take punitive action in case of non-compliance, furnishing of wrong information or in case of any damage to the environment.
Procedure to accord approval:
- laboratory and greenhouse experiment,
- open field trials for generation of biosafety data,
- commercialization and market approval
- Large scale production.
- International conventions related to this:
- The committee report passes references to all these conventions.
Convention on Biological Diversity (CBD)
- CBD is a legally binding agreement adopted during Rio Earth Summit in 1992. India signed the CBD and ratified Convention on biological diversity. USA is not a party to this Convention.
- While reaffirming sovereign rights of nations over their natural resources, this Convention establishes three goals: conservation of biological diversity, sustainable use of its components, and fair and equitable sharing of benefits arising from the use of genetic resources.
- India is hosting CoP-11 to the CBD to be held in Hyderabad in October 2012, which is the year of 20th anniversary of Rio Earth Summit is an added information.
Cartagena Protocol on Biosafety (CPB)
- The CPB, the first international regulatory framework for safe transfer, handling and use of LMOs(living modified organisms) signed on 2000. India has acceded to this Biosafety Protocol.
- Difference between LMO and GMO is that GMO is a broader term including LMOs within it, all organisms live, dead whose genes are modified are GMOs but LMO as the name suggests are living organisms which are genetically modified.
- The objective of the Protocol is to contribute to ensuring an adequate level of protection in the field of the safe transfer, handling and use of LMOs resulting from modern biotechnology that may have adverse effects on the conservation and sustainable use of biological diversity, taking also into account risks to human health, and specifically focusing on trans-boundary movements.
- As a Party to the Protocol, the first and foremost requirement is the setting up of a National Biosafety Regulatory Framework India has introduced the national biosafety rules even before the Convention on Biological Diversity (CBD) was adopted at Rio de Janeiro in 1992.
- Even though the text of the Protocol has been adopted, several critical issues such as risk assessment, liability and redress, documentation and identification of LMOS for Food Feed and Processing etc., are still under discussion.
Nagoya Protocol on Access and Benefit Sharing (ABS)
- The CoP-10 to the CBD held in Nagoya, Japan in October 2010 adopted the Nagoya Protocol on Access and Benefit Sharing .India is a megadiverse country rich in biodiversity and associated traditional knowledge. Hence, implementation of the ABS provisions of CBD is of special interest to us.
- The objective of Nagoya Protocol is the fair and equitable sharing of benefits arising from utilization of genetic resources. The Protocol establishes a clear framework on how researchers and companies can obtain access to genetic resources and to associated traditional knowledge, and how benefits arising from the use of such material or knowledge will be shared with locals.
- The ABS Protocol is expected to address the concerns of biodiversity rich countries such as India relating to misappropriation of genetic resources and associated traditional knowledge.
This was a guest article by Manikandan Soundararajan
Reference: “CULTIVATION OF GENETICALLY MODIFIED FOOD CROPS – PROSPECTS AND EFFECTS”-BASUDEB ACHARIA (chairman)

![[Laws] DESI liquor special edition Kerala, Mizoram & IPS Training, Tribal insurgency](https://mrunal.org/wp-content/uploads/2014/09/Cover_Polity_kerala-liquor-500x383.gif)
![[Economic Survey] Corrections in the previous articles + Parting words before Qatl ki Subha](https://mrunal.org/wp-content/uploads/2014/08/Cover-Economic-Survey-correction-500x383.jpg)
Just a quick clarification regarding “TRANSGENICS, transformed genetic make-up.”
Transgenics are specifically organisms which have genes from some other species. The definition given in the article is for genetically modified organisms.
For E.g. Bacteria used to produce insulin are inserted with gene responsible for insuling production if I recall correctly.
DNA are actually made of nucleotides which are made of nucleobases called A,T,G,C. Genes are sections of DNA in most cases and for many virus sections of RNA.
mr. mnural
i appreciate your effort to make the whole thing so lucid and comprehensive ,but i would like to bring your attention that the definition mentioned “DNA is double stranded particle made of proteins” is completely wrong. please correct it.
DNA is a polymer of nucleic acid specifically deoxyribonucleic acid which is in turn is comprised of sugar component and nitrogenous base.there are 4 type of nitrogenous base that is adenine , guanine, cytosine and thiamine. It is the sequence of theses nitogenous bases that determine our genetic charecter.
thanks
ritesh
this is the supplemented protocol of nagoya and kolalumpper, cartagena protocol on genetically modifed food .
Sorry friends one correction. My intention was to express DNA aids in protein synthesis. DNA are actually made of a pentose sugar called deoxy ribose with phosphates. It is this pentose sugar which in itself is bound 4nucleotide bases adenine,guanine, thymine,cytosine.Two strands of these deoxy ribose,phosphates, nucleotide bases are present as a double helical strand bound together by hydrogen bonds. This DNA molecule with unique arrangements of nucleotide bases encode protein for various expressions. This arrangement depends on our ancestry. This protein synthesis happens thru two processes called transcription and translation. The sentence in I st para should have been DNA are essential in making of proteins.
Hi Mrunal,
GEAC is Genetic Engineering Approval Committee, not ‘Appraisal’ committee
Cheers!
Hi .
They say BT Brinjal is the first edible GM crop in India , whereas what about the High yielding varieties introduced under Green Revolution .
can someone please explain ?
Hi ah. tilak ji
HYV UNDER GREEN REVOLUTION WERE CROSSED WITH HYV OR THROUGH SUCCESSIVE SELECTIONS WITHOUT CHANGING GENETIC CODE[DNA][SEQUENCES OF NUCLIC NITOGENOUS CHANGES TO CHANGE HEREDITARY CHARACTERS ]
NATURALLY BIG GUNS WILL PLAY TO INITIATE TRANSGENIC SEEDS MARRING POOR FARMERS RIGHT[VIDE FARMER,S RIGHT IN VIEW OF IP RIGHT]THIS SITUATION MAY COMPEL HIM TO SUICIDE.
THUS IT IS SENSITIVE MATTER TO BE LOOKED INTO FROM ALL ANGLES.
It should be ‘Thymine’ & not ‘Thiamine’…
What is mean by Genetic coloniyalisam ?
Bt. brinjal can prevent condition like farmers getting intoxicated by coming under insecticide spray exposure. Cotton used to be one such crop before 2002 which accounted for about 50 % of insecticide consumption in India but after the introduction of Bt cotton not only farmers were relieved but also there was staggering rise in cotton trade( because of less insect pests incidence) from meager 51 crores in 2000-2001 to 10241 crores in 2007-08. Now the whole vegetable fields are in-fluxed with insecticide, bringing in Bt brinjal would not only help consumers to get residue free food but also farmer in getting more yield
Bt brinjal is brought to control Fruit and Shoot borer , Leucinodes orbonalis where as Bt cotton with cry 2 gene is to control 4 types of lepidopterous larvae such as Helicoverpa armigera, Spodoptera litura, Earias Vitella and Pectinophora gossypiella
Future
Cartagena= BTRA Bill, 2013
Nagoya= intro SRI, then HYDCBD